Magstim gains FDA Clearance for TMS Treatment in U.S. Adolescent Patients
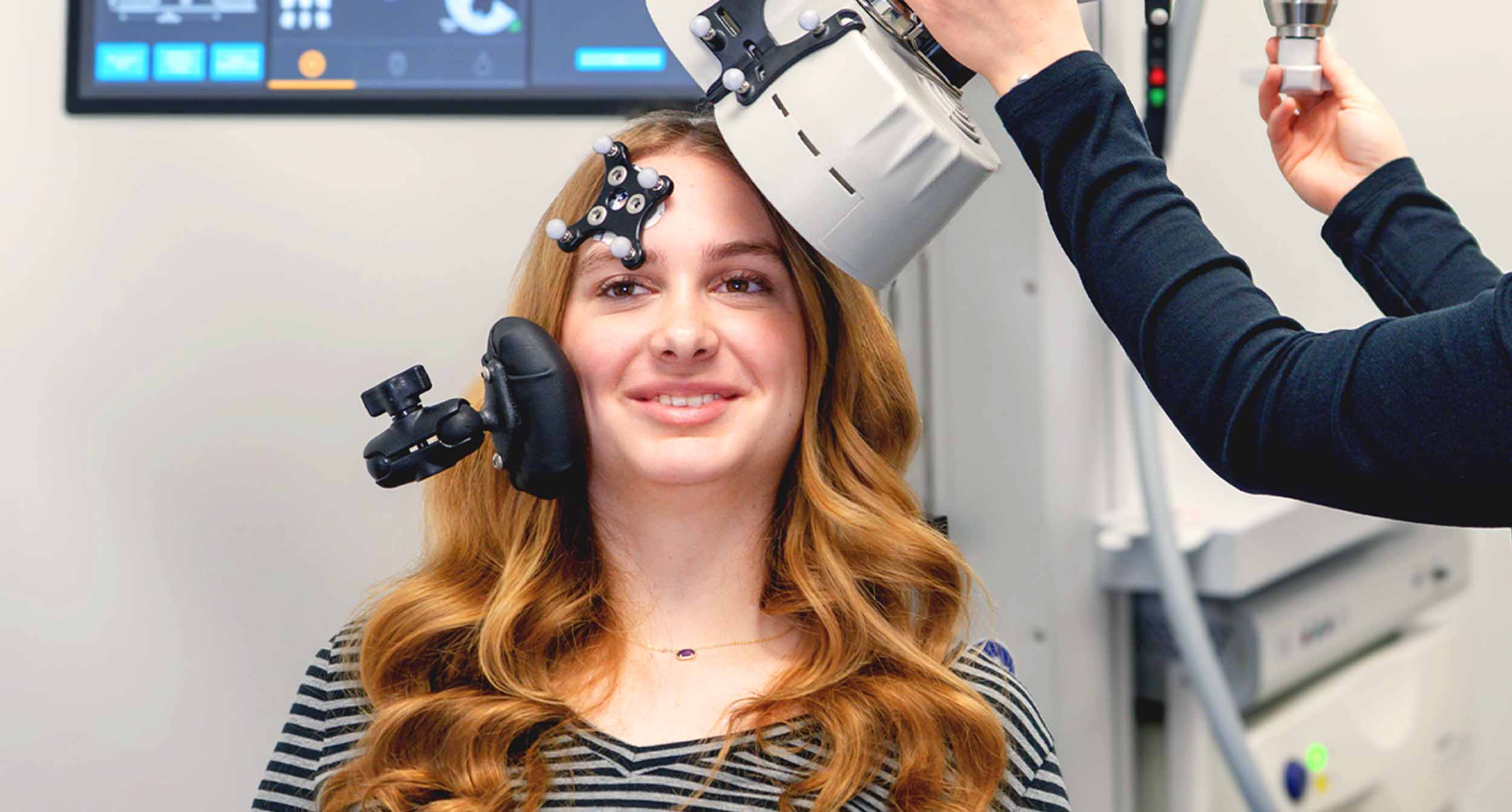
Magstim Horizon Inspire and 3.0 Transcranial Magnetic Stimulation Systems have received FDA clearance for the treatment of Major Depressive Disorder (MDD) in adolescent patients, ages 15-21.
This represents a major advancement in mental health care, introducing new opportunities for effective, non-invasive treatments designed specifically for younger patients struggling with depression.
To read the full article about Magstim - click here.
To learn more about the Horizon 3.0 with StimGuide Pro, please watch our new video, click here.
To learn more about the Horizon Inspire, please watch our new video, click here.

